THE NANOPDICS PROJECT·····
ION Channels
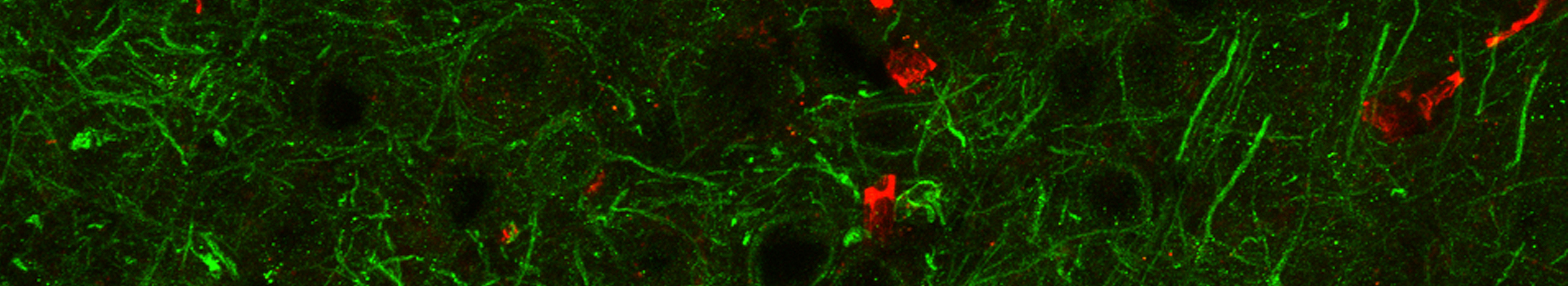
Many cell types in living systems use electrical signaling to perform their physiological functions. Cells store and deploy electrical energy to, among others, communicate, release hormones or neurotransmitters, grow and divide. Ion channels are intriguing proteins located that allow the controlled flow of specific ions (potassium, sodium, calcium, magnesium, chloride…) across cellular membranes. The amazing thing about ion channels is that their function can be studied in real time, using techniques like patch-clamp and fluorescence. Not surprisingly, malfunction of these proteins constitutes the common cause of many diseases, including epilepsy, migraine, muscular dystrophy, hypertension, etc. Due to their unquestionable physiopathological relevance, ion channels are nowadays one major pharmaceutical target.
BK Channels
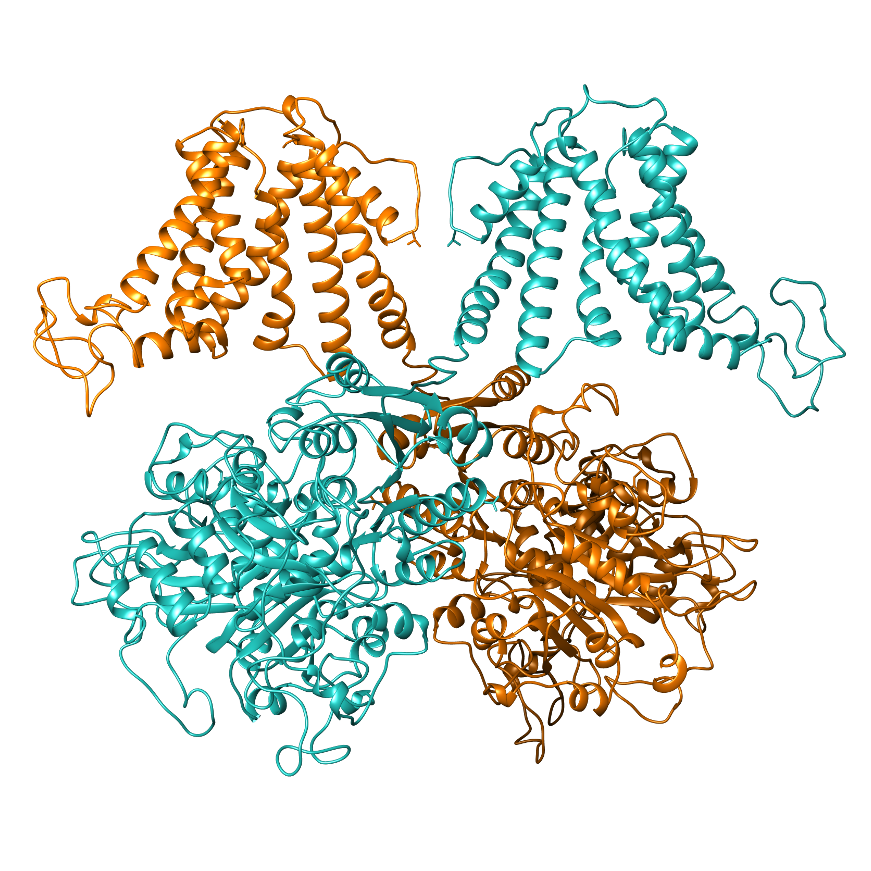
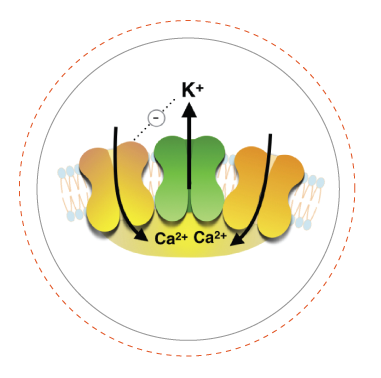
One important focus of our lab are large conductance calcium- and voltage-gated potassium channels (also known as BK, slo or MaxiK channels), which are able to regulate potassium flow in response to intracellular Ca2+ and membrane voltage signals. This intrinsic property makes them essential players in vital physiological processes where intracellular Ca2+ signaling is coupled to control of membrane voltage, such as neurotransmission, endocrine or muscle function. In the nervous system, the interaction between Ca2+ influx through voltage-gated calcium channels and BK activation is involved in numerous essential neuronal processes such as repolarization and hyperpolarization following the action potential (AP), dendritic Ca2+ spikes, and neurotransmitter release. Inherited defects in BK channels function lead to seizure and epilepsy, indicating that this coupling mechanism is crucial to regulate neuron excitability in the healthy brain.
OTHERS PROJECTS IN THE LAB······
Kv7 Channels
In neurons, voltage-gated potassium channels formed by Kv7.2 and Kv7.3 (KCNQ2 & 3) subunits constitute the molecular basis of the neuronal M-current and are key players in the control of the neuronal membrane resting potential, constituting a potent inhibitory mechanism in the brain. In fact, it has been reported that even a slight reduction in Kv7.2/3 function can be epileptogenic. The identification of additional mechanisms regulating Kv7 channels is one of the most ambitious goals in today’s anti-epileptic research. Our lab has discovered that a novel neuronal isoform of the serum and glucocorticoids regulated kinase 1, namely sgk1.1, upregulates the Kv7.2/3 heterotetramer complex, by increasing the number of channels at the neuronal membrane. The transgenic mice generated in our lab, expressing a constritutively active form of sgk1.1, are protected against convulsions, opening a new door towards a novel M current regulator. Among other things, we are working in understanding where and how does this protection occur in the brain, and how to directly modulate sgk 1.1 function.
Epithelial sodium channels (ENaC)
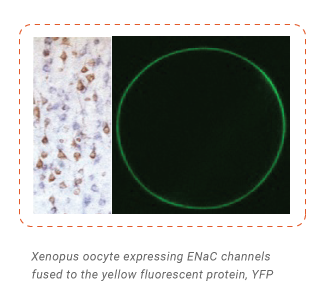
The epithelial sodium channel (ENaC) constitutes the rate-limiting step for sodium transport across electrically tight epithelia. These channels are formed as trimers of alpha, beta and gamma subunits. Regulation of ENaC activity is critical for electrolyte and extracellular volume homeostasis, as well as for lung liquid clearance and colon sodium handling. In our lab we study the molecular composition, functional properties and possible physiological roles of voltage-independent sodium channels formed by the ENaC delta subunit. This intriguing subunit presents atypical characteristics, since unlike classical ENaC subunits, is expressed in non-epithelial tissues. Its coding gene is present only in human and primates.
Our lab is proudly funded by